In a latest research printed within the journal Npj Vaccines, researchers introduced the tactic of Reverse Vaccine Improvement, which offers a possibility to find out the correlates of safety within the early phases of scientific trials for vaccines in opposition to pathogens which might be proof against antimicrobial brokers to stop issues reminiscent of important phase-III scientific trial failures, lack of funding in vaccine improvement, and populations being uncovered to ineffective vaccines.
 Perspective: Reverse improvement of vaccines in opposition to antimicrobial-resistant pathogen. Picture Credit score: Kateryna Kon / Shutterstock
Perspective: Reverse improvement of vaccines in opposition to antimicrobial-resistant pathogen. Picture Credit score: Kateryna Kon / Shutterstock
Background
The event of antimicrobial resistance in pathogens is quickly turning into a public well being concern of the identical or probably larger magnitude as malaria or human immunodeficiency virus (HIV). Nonetheless, the method of creating vaccines is tedious and costly, and within the case of antimicrobial-resistant pathogens, it’s made worse by insufficient info on correlates of safety.
Within the case of extreme acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the method of vaccine improvement was considerably accelerated by the invention of antibodies that would bind to the spike protein of the virus, stopping it from binding to the host angiotensin-converting enzyme-2 receptor. This discovery additionally indicated that neutralizing antibody titers might be used as correlates of safety since they indicated the scientific efficacy of the vaccine.
For many antimicrobial-resistant pathogens, the mechanisms by way of which vaccines can shield the host stay unknown. Whereas immunomics, proteomics, and genomics are being extensively used to develop vaccines in opposition to antimicrobial-resistant pathogens, the dearth of data on correlates of safety continues to current the chance of jeopardizing late-stage scientific trials.
In regards to the research
Within the current research, the researchers introduced a technique of Reverse Vaccine Improvement, a brand new paradigm for vaccine improvement that requires info on the efficacy of the vaccine and the immune responses to be generated a lot earlier within the vaccine improvement course of in order that the correlates of safety will be recognized early on as an alternative of nearer to section III trials. In addition they applied this paradigm to guage a vaccine in opposition to the antimicrobial micro organism Staphylococcus aureus.
The method is known as Reverse Vaccine Improvement because the order of data procurement on vaccine efficacy is reversed as in comparison with the standard process of vaccine improvement. This info is obtained from populations which might be already experiencing a excessive incidence of antimicrobial-resistant pathogenic infections as an alternative of the inhabitants that can ultimately get vaccinated.
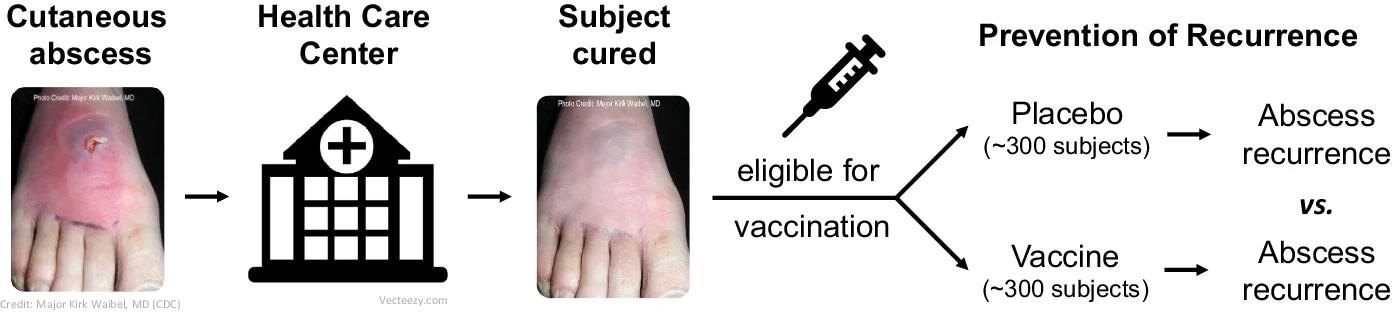
Paradigm scientific trial design (section 1/2) in Reverse Vaccine Improvement: S. aureus vaccine in topics at excessive threat of SSTI.
On condition that animal fashions haven’t been unreliable in vaccine improvement in opposition to antimicrobial-resistant pathogens, utilizing high-risk populations helps examine the immune responses of unprotected and guarded people, which may present information on correlates of safety.
The efforts to develop vaccines in opposition to S. aureus have yielded 4 candidate vaccines that focus on varied antigens and use 4 completely different safety mechanisms. Primarily based on the outcomes from animal mannequin research and in-vitro assays, the vaccines have been superior to section I and II scientific trials. The vaccines handed the protection assessments and elicited passable antibody titers. Nonetheless, the section III efficacy trials for these vaccines failed, indicating an absence of satisfactory info on correlates of safety.
To bypass such issues, the researchers on this research utilized the paradigm of Reverse Vaccine Improvement to design a randomized, observer-blinded, placebo-controlled section I and II trials to evaluate the immunogenicity, security, and efficacy of the candidate vaccine developed in opposition to S. aureus by GSK.
Outcomes
The research mentioned how Reverse Vaccine Improvement differs from the normal vaccine improvement course of by starting in section I or II trials that consider the efficacy, immunogenicity, and security of the vaccine as an alternative of efficacy analysis in section III trials. This ensures that potential issues related to correlates of safety are recognized early within the vaccine improvement course of and don’t end result within the failure of the vaccine in the direction of the tip phases when appreciable sources have been invested within the course of.
Section I security trials with and with out adjuvant are sometimes carried out if the vaccine is being developed for the primary time for people, and primarily based on the outcomes of the section I security assessments, the trial proceeds into section II to guage the efficacy and immunogenicity. Evaluating the immune responses elicited by the vaccine amongst unprotected and guarded teams can assist determine correlates of safety, which may then be used to formulate, schedule, and facilitate vaccine efficacy assessments within the common populations and refine the vaccine dosage.
The researchers mentioned intimately the varied parameters that have to be evaluated when correlates of safety are being explored. These included serology, mobile responses, immunological alerts, transcriptional profiles, reminiscence immune cell responses, and background immunity.
Conclusions
To summarize, the research described a novel vaccine improvement paradigm that entails conducting section I and II trials in populations which might be at excessive threat of contracting the goal antimicrobial-resistant pathogen to grasp the correlates of safety earlier than the event course of progresses to section III trials and dangers failure. This methodology might circumvent grave issues in vaccine improvement, reminiscent of publicity to inefficacious vaccines and the lack of useful resource funding.
Journal reference:
- Bagnoli, F., Galgani, I., Kumaran, V. V., & Phogat, S. (2024). Reverse improvement of vaccines in opposition to antimicrobial-resistant pathogens. Npj Vaccines, 9(1), 71. 10.1038/s41541024008584, https://www.nature.com/articles/s41541-024-00858-4
